Techniques for the Determination of Clutch Parameters in Darters and Minnows: A Proposal for Standard Methods by David C. Heins
Clutch size, egg size and clutch mass are important life history traits which are frequently measured for animals. The accurate determination of these clutch parameters is of critical importance in the study of life history evolution. Accurate and precise techniques, including standardized methods, allow researchers to benefit from the collective efforts of the scientific community in making broad comparisons based on studies of limited scope.
Historically, biologists studying minnows and especially darters have held two fundamentally different views about the processes by which ova are produced and spawned. The methods that have been adopted by individual researchers for the study of clutch parameters have been a reflection of these two views and as such differ greatly in the data that have been generated using them. Thus, two very different sets of techniques have been used to determine the clutch parameters of both darters and minnows in past studies. Because the two techniques are used to provide the same data and are based on completely different ideas of ovum production, both cannot be correct.
Presented here is a proposal for standard methods, which are based upon recent studies of ovum production in darters and minnows. These studies have important implications for different methods used for the study of life history traits. The techniques presented here are, therefore, based upon published results and appear to represent the approach, which will yield accurate and comparable data. Nonetheless, these methods may be modified as additional information becomes available.
During the spawning season, sexually mature females often, if not usually, have ovaries containing gametic cells, which comprise two distinct groups. These two groups of cells differ in size, color, opacity and transparency. The most important of these characteristics is size because it is the characteristic by which the two groups always can be separated.
The group of smaller cells appears much the same from one female to the next, being opaque and varying in color from white to cream to yellow or even dark yellow. The group of larger cells will vary greatly in appearance from female to female, depending on each female's reproductive condition. They may be opaque and yellow to dark yellow or sometimes even orange. In other cases, they may be translucent to transparent and yellow to amber or orange. Still other characteristics, which are discussed below, may be used to distinguish the stages in the formation of eggs.
Names applied to the gametic cells in the ovaries of fishes have varied among authors. One common approach has been to refer to all the cells as "ova" or "eggs" and to distinguish among them by using adjectives to describe the stage of formation, e.g. "immature ova" and "mature ova." To allow greater precision in the description and/or study of reproductive biology, modification of this nomenclature is needed, as shall be addressed later.
Using Only "Mature Ova"
This technique is based upon the hypothesis that females produce only one clutch of eggs each reproductive season. Researchers who have adopted this technique have counted and measured only the relatively large "mature ova" in the ovaries of preserved females, under the assumption that they represent the single clutch of eggs to be produced in that year.
Just which intra-ovarian cells comprise "mature ova" is not always defined or defined with sufficient specificity to allow other researchers to determine exactly which cells were counted and measured. A composite list of descriptive characteristics from an umber of reports gives the following general description of "mature ova": transparent or translucent, yellow to orange, one or more oil droplets, chorions elevated, occur in ripe females, tend to be concentrated near center of ovary, and easily detached from surrounding tissue.
Using Both "Immature Ova" and "Mature Ova"
Under the assumption that females produce more than one clutch of eggs each reproductive season (year), some researchers have counted the relatively small "immature ova" in addition to the larger "mature ova," representing all easily visible gametic cells within an ovary. This approach also is based on two additional assumptions. First, that all cells within the ovaries of each female are spawned in an undetermined number of clutches during the course of one reproductive season; Second, that no additional cells begin development during the reproductive season; therefore, the maximum possible number of eggs is set at the beginning of the reproductive season.

Both former techniques for the determination of clutch parameters have inherent problems because neither is based on a full understanding of the reproductive biology of these fishes. Moreover, the dichotomy of "immature" and "mature" eggs is not detailed enough to describe the entire process of ovum formation, hence, clutch production. As a result, structures in more than one stage of a more refined classification system may be used, resulting in significant errors in determinations of clutch parameters. The central question is: which ovarian cells does one count and measure to obtain accurate estimates of clutch parameters? The answer lies in an understanding of the ovum development process.
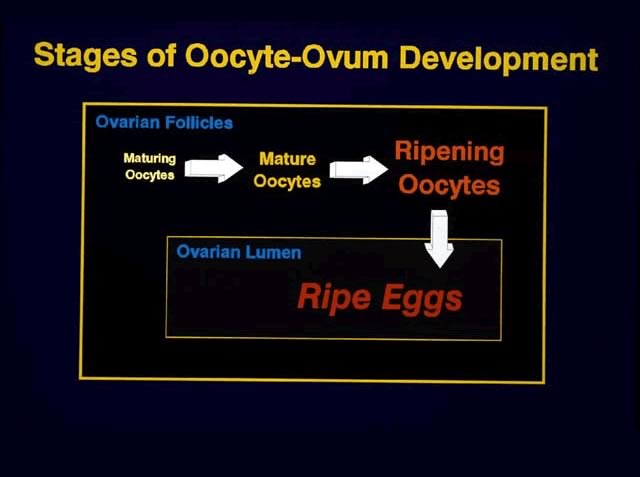
As the spawning season of a species nears, sexually mature, adult females begin the formation of eggs. In doing so, they progress from the latent (LA) stage through early maturing (EM) and late maturing (LM) stages of ovarian condition. Throughout this time only one group of oocytes is present in the ovaries, although the group often is bimodal in its size distribution in LM females. This group of oocytes is a recruitment stock from which successive clutches are formed.
As some, but not all, oocytes in LM females undergo vitellogenesis (yolk loading), they form a second, distinct group of larger oocytes, in the follicles. The entire group of oocytes progresses through three major stages of development to form the eggs (clutches) that will be spawned. Both the oocytes/eggs in the group of larger cells and the ovaries are classified using the same categorical terms. Thus, this discussion will focus on the changes in the group of larger oocytes, which represents the developing clutch.
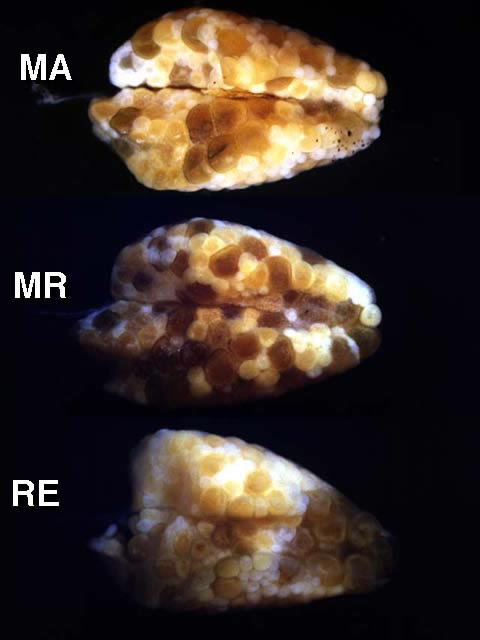
In chronological sequences, mature oocytes are initially found in mature (MA) females. The mature oocytes later undergo final oocyte maturation as yolk loading ceases and are at that time referred to as ripening oocytes, which are found in ripening (MR) females. (MR stands for mature ripening.) The ripening process involves the hydration of the follicles, which eventually will burst, releasing ripe eggs into the ovarian lumina of the ripe (RE) females.
One should keep in mind that MA, MR, and RE females also have another group of gametic cells in their ovaries. These are the smaller oocytes, which we refer to as maturing oocytes, and which form the recruitment stock of oocytes from which groups of oocytes are drawn successively to produce clutches. Thus, after a group of vitellogenic oocytes has become separated from the recruitment stock of smaller, maturing oocytes, the oocytes themselves progress through three distinct stages of development during the formation of a clutch: mature oocyte, ripening oocyte, and ripe egg.
In RE females collected soon after ovulation, the ripe eggs will fill the centers of the ovaries and the maturing oocytes will form a layer around them. The ripe eggs will be concentrated at the posterior end of the ovary in females that have spawned a percentage of their eggs. Both maturing and mature or ripening oocytes, respectively, are present in the individual follicles of mature and ripening females. Thus, structures in different stages are interspersed among one another. Moreover, the individual oocytes must be extracted from the follicles and cleaned off to remove all follicular remnants. In ripe females, the ripe eggs are clustered like grapes and can easily be separated from one another after peeling the maturing oocytes away from the clump.
Data from field and laboratory studies have shown that females do not necessarily stop reproducing after producing just one clutch. Instead they are capable of producing multiple clutches as they cycle through the LM, MA, MR, and RE stages of ovarian condition. This cycle has been called the "clutch production cycle." Thus in a single collection taken during the spawning season, adult females may be found in one or more of the clutch production stages: LM, MA, MR and RE stages. All four stages will not necessarily be present in any one collection. LM females will have but one group of oocytes, although the group usually will show a bimodal size distribution. MA, MR, and RE females will have two easily separable size groups of gametic cells, although color, opacity, and transparency also may differ between the groups: maturing oocytes plus either mature oocytes, ripening oocytes, or ripe eggs.
As the close of the reproductive season draws near, females begin resorbing the oocytes in their follicles and become LA. This process usually seems to begin in LM and MA females.

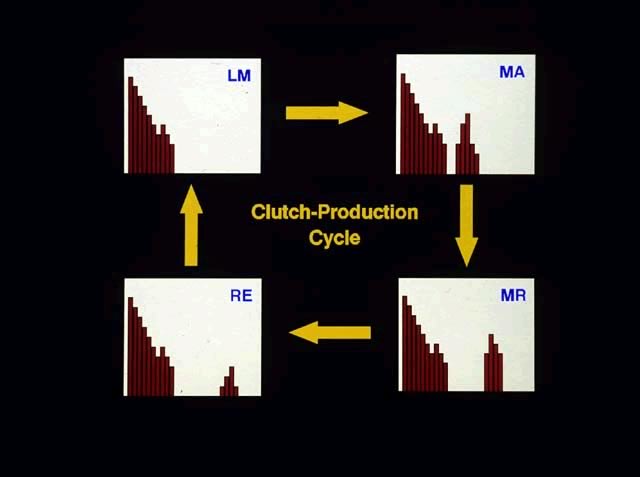
Clutch Size
A clutch is a group of eggs, which is formed from synchronously developing oocytes and is oviposited within a relatively short interval of time. One of the goals in studying fecundity should be to determine the sizes and frequency of clutches produced during a spawning season. Results from a number of studies have shown that all mature or ripening oocytes in an ovary are ovulated as a group to produce a clutch of ripe eggs which is then spawned (oviposited) over a relatively short period of time whether all at once (e.g. Gasterosteus aculeatus) or in successive bouts as in many darters and minnows.
Because females begin spawning soon after ovulation, we generally expect the ovaries of RE females to contain fewer eggs than the number they typically spawn from a complete clutch. Data show that RE females yield spurious estimates of clutch size and also will result in erroneous statistics for clutch size body size relationship.
One therefore should use only MA or MR females to estimate clutch size. At times, one to a few ripening oocytes from a previous clutch will be present in the follicles of MA females. One should not count them. Ripening oocytes that are not ovulated with the rest of their clutch appear to be resorbed; they do not appear to be ovulated with the next clutch.

Ovum Size: Diameter and Mass
Ripe eggs provide the best estimates of egg size mass or diameter because they represent the final stage of development. Ova removed from females as well as spawned ova may be used. One should be careful to identify the stage of the ova (ovulated from female vs. spawned) as diameter measurements will be affected. In general, spawned ova should have greater diameters ripe ova removed from females due to further hydration of the eggs after oviposition.
Ripening oocytes provide a good estimate of ovum mass as yolk loading has ended by this stage of ovum formation. One should be sure that all follicular material has been removed from the oocytes, as ripening oocytes can be difficult to clean. Diameters of ripening oocytes will underestimate the diameters of ripe eggs in females because they are not yet fully hydrated.
Both the mass and the diameter of ripe eggs in females will be underestimated by using mature oocytes. Mature oocytes may, however, serve as useful indices of the size of ripe eggs for purposes of comparisons, although this has not yet been demonstrated conclusively. At present, data seem to suggest that in some cases one might not detect differences in ripe eggs by using mature oocytes due to greater variability in mature oocytes. In all cases, the use of oocyte/ovum dry mass is preferable to diameter as a measure of nutritive material afforded the young. Correlations between oocyte/ovum diameter and mass decrease sequentially between mature oocytes, ripening oocytes, and ripe eggs due at least in part to the hydration of the latter two stages. Thus, diameter is not as good a predictor of mass in ripening oocytes and ripe eggs as in mature oocytes.

Relative Clutch Mass (RCM)
One approach to estimating relative clutch mass would be to use the mean and variance for the mass of individual ripe eggs from RE females and the clutch sizes of MA and MR females to estimate clutch mass just before spawning. The MA and MR females would, therefore, be dried and weighed to use the body weights to estimate RCM. Clutch and body weights from MA females may provide indices of RCM in females just prior to spawning but there are no data addressing this question. MR females should provide the most straightforward estimates of RCM, but they are often rare in collections.

Drying Temperatures
The temperature at which oocytes, ova, and carcasses are dried will affect the masses of the tissues being weighed. In general, there is a sequential loss of lipids at higher temperatures. Thus, one should dry tissues at the lowest possible stable oven temperature. Many ovens cannot maintain a stable temperature below about 40°C and others are limited below 30°C. Thus, 30-40°C is the recommended temperature range. The oven should be able to maintain a very narrow range of temperature variation. A suggested maximum range of variation is ±1°C.
Weighing Tissues
Transfer tissues from the oven to a dessicator with fresh dessicant as rapidly as possible to avoid allowing the tissue to take up moisture. The balance chamber should also have a dessicant in it. The transfer of tissue from the dessicator to the balance should also be as rapid as possible.
This information focuses on determinations of clutch size and oocyte/ovum diameter measurements. Relative clutch mass and oocyte/ovum mass rarely have been determined for minnows and darters.
Methods associated with the single clutch hypothesis did not provide a definition for "mature ova" which was specific enough to limit the use of oocytes or ova in one stage of development. Most aspects of past descriptions appear to indicate that ripe eggs (present terminology) were used for determinations of both clutch size and ovum size. In some cases, there is a possibility that ripening oocytes also were used in obtaining estimates of these parameters.
The methods proposed here advocate counting the oocytes in clutches of MA and MR females before the oocytes are ovulated. Females begin spawning soon after ovulation. Thus, the use of ripe eggs (from RE females) will result in underestimates of clutch size, which may be quite large, and also will yield spurious regression statistics. Diameter measurements of ripe ova alone will be comparable with other similar measurements. If ripe eggs and ripening oocytes were measured the data cannot be compared without error unless it is possible to restage the cells, which were measured. In cases where the specimens and the ovarian material remain in collections, this may be possible.
Methods formerly associated with the multiple clutch hypothesis involved counting all gametic cells, including maturing oocytes (present terminology) and any mature or ripening oocytes or ripe eggs (present terminology). The assumption was that this complement represented all of the eggs to be spawned in one season. Research has shown that individual darters and minnows are capable of producing a much greater number of ova in one season than the number of all gametic cells present at any one time. Thus, counting all gametic cells has very little meaning in relationship to the number of ova spawned within one season. Similarly, measurements of diameters of gametic cells in these stages of ovum formation are likely to have little relevant meaning. Again, the methods advocated here for determining clutch sizes involve making counts of oocytes in a clutch under formation in MA and/or MR females before the clutch is ovulated. Estimates of ovum size ideally should be obtained using the masses of ovulated eggs from RE females. The difficulty is that RE females are not always available when one obtains samples, suggesting that the process of oocyte ripening and ovulation occurs over a relatively short period of time.
In summary, the methods proposed here provide a snapshot of reproduction at one point in time. The more difficult task is to estimate clutch frequency and to factor variations in clutch size into any studies one is conducting. Similarly, variation in ovum size among clutches must be considered. Although both measurements of diameter and mass are useful, measurements of mass are of greater utility. Ideally, measurements should be completed using ripe eggs; however, limitations associated with the availability of ripe females may force one to use mature or ripening oocytes, which vary in the nature of the data they yield.
The limited information available shows that the methods of fixation and preservation used can have significant effects on the results one obtains. Alcohol can remove lipids from tissues, including oocytes and ova during storage. At present, one is best advised to fix specimens in 10% formalin and to store specimens in either 3%, 5% or 10% formalin.
Baker, J.A., and D.C. Heins. 1994. Effect of drying temperature on weight estimates for oocytes and eggs in the darter Etheostoma lynceum. Copeia 1994: 821 – 823.
Gale, W.F., and G. Buynak. 1978. Spawning frequency and fecundity of satinfin shiner (Notropis analostanus) a fractional, crevice spawner. Trans. Am. Fish Soc. 107: 460 – 463.
Gale, W.F., and W.G. Deutsch. 1985. Fecundity and spawning frequency of captive tessellated darters fractional spawners. Trans. Amer. Fish. Soc. 114: 220 – 229.
Gale, W.F., and C.A. Gale. 1977. Spawning habits of spotfin shiner (Notropis spilopterus) — a fractional, crevice spawner. Trans. Amer. Fish. Soc., 106: 170 – 177.
Goetz, F.W. 1983. Hormonal control of oocyte final maturation and ovulation in fishes, p. 117–170. In: W.S. Hoar, D.J. Randal and E.M. Donaldson (eds.). Fish Physiology, Volume IX, Part B. Academic Press, New York.
Heins, D.C., and J.A. Baker. 1988. Egg sizes in fishes: do mature oocytes accurately demonstrate size statistics of ripe ova? Copeia 1988: 238 – 240.
Heins, D.C., and J.A. Baker. 1993a. Clutch production in the darter Etheostoma lynceum Hay and its implications for life history study. J. Fish Biol. 4: 819 829.
Heins, D.C., J.A. Baker, and W.P. Dunlap. 1992. Yolk loading in oocytes of darters and its consequences for life history study. Copeia 1992: 404 – 412.
Heins, D.C., J.A. Baker, R.W. King, and K. Lahti. 1996. Evaluation of ovum fixation and storage in threespine stickleback Gasterosteus aculeatus. J. Fish Biol. 00:000 – 000.
Heins, D.C. and M.D. Machado. 1993. Spawning season, clutch characteristics, sexual dimorphism and sex ratio in the redfin darter Etheostoma whipplei. Am. Midl. Nat. 129: 161 – 171.
Hubbs, C. 1983. Handbook of darters (review). Copeia 1983: 581.
Hubbs, C. 1985. Darter reproductive seasons. Copeia 1985: 56 – 68.
Hubbs, C. and K. Strawn. 1957. The effects of light and temperature on the fecundity of the greenthroat darter, Etheostoma lepidum. Ecology 38: 596 – 602.
Wallace, R.A. and K. Selman. 1981. Cellular and dynamic aspects of oocyte growth in teleosts. Am. Zool. 21: 325 – 343.
Weddle, G.K. and B. M. Burr. 1991. Fecundity and the dynamics of multiple spawning in darters: an in stream study of Etheostoma rafinesquei. Copeia 1991: 419 – 433.
Mature Oocyte
Un-ovulated, cream to yellow, yolk opaque to sometimes slightly translucent, chorion not separated from yolk mass. Found in follicles of MA females.
Ripening Oocyte
Un-ovulated, yellow to amber, yolk becoming translucent to transparent, chorion slightly to moderately separated from yolk mass. Found in follicles of MR females.
Ripe Egg
Ovulated, yellow to amber, yolk translucent to transparent, chorion moderately to usually well separated from yolk mass. Found in lumens (central cavities) in ovaries of RE females. May appear to be concentrated in the posterior portion of the ovaries.
Stages of ovarian condition in adult darters based upon Heins and Rabito (1986), Heins and Baker (1989), and Heins et al. (1992).
Stage: Latent (LA)
Ovaries very small, thin, and transparent to slightly translucent. Maturing oocytes, if present, yolkless or vitellogenic with nucleus visible.
Stage: Early Maturing (EM)
Ovaries small to moderate in size and translucent to white (opaque). Maturing oocytes small to moderate size and translucent to white with nuclei obscured by yolk.
Stage: Late Maturing (LM)
Ovaries small to greatly enlarged and white to cream. Maturing oocytes moderate to large size and white to cream.
Stage: Mature (MA)
Ovaries moderate sized to greatly enlarged and cream to yellow. Two separate groups of follicular oocytes: a group of smaller, maturing oocytes, and a group of larger, mature oocytes which are opaque (usually) and cream to yellow without chorions separated from the yolk.
Stage: Ripening (MR)
Ovaries moderate sized to greatly enlarged and cream to yellow. Two distinct groups of follicular oocytes. The larger, ripening oocytes are translucent (most often) to transparent with chorions separated from the yolk.
Stage: Ripe (RE)
Ovaries moderate sized to greatly enlarged and cream to yellow. One group of maturing follicular oocytes, which are moderate to large sized and white to cream. Ripe ova concentrated in the lumen of the ovary (usually posterior) and translucent to transparent (usually) with chorions separated from the yolk.
Figure 1
Ventral view of ovaries from mature (MA) female of Etheostoma caeruleum. Two entirely separate groups of oocytes are present in the follicles: smaller maturing oocytes and larger mature oocytes. A histogram showing a plot of oocyte size versus percentage frequency of occurrence from the ovaries would show to entirely separate groups of oocyte. Size is the primary criterion as color may vary. A female (ovaries) with but one group of oocytes even if bimodal would be classified as late maturing (LM).
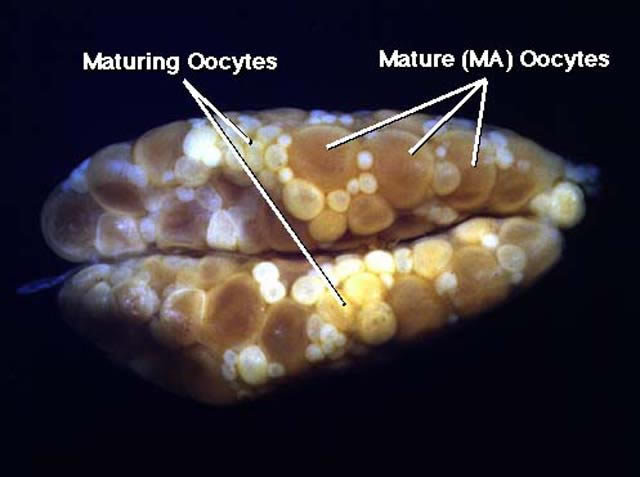
Figure 2
Ventral view of ovaries of mature (MA) female of Etheostoma caeruleum. In addition to smaller maturing oocytes, a group of larger maturing oocytes is present in the follicles. The mature oocytes are opaque, and the chorions are not elevated.
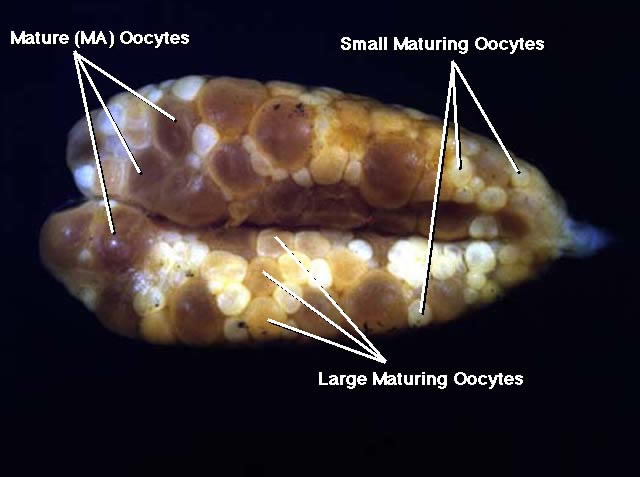
Figure 3
Ventral view of ovaries of ripening (MR) female of Etheostoma caeruleum. The group of larger gametic cells, which is present in the follicles are ripening oocytes undergoing final oocyte maturation. The ripening oocytes are translucent to sometimes transparent and the chorions are elevated from the yolk mass.

Figure 4
Ventral view of ovaries of ripe (RE) female Etheostoma caeruleum. Only smaller maturing oocytes are present in the follicles. The larger gametic cells are ripe eggs which are ovulated and occupy the lumina in the centers of the ovaries. The ripe eggs are translucent to transparent and have elevated chorions. The eggs are lumped together. Thus, they only need to be separated from one another after the entire man is separated from the maturing oocytes. Mature and ripening oocytes must be extracted from the individual follicles and are distributed among the smaller maturing oocytes.
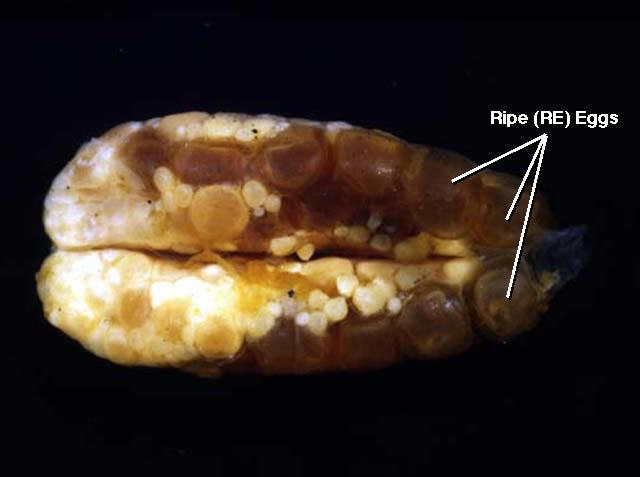
Figure 5
Ventral views of ovaries from mature (MA), ripening (MR), and ripe (RE) females of Etheostoma caeruleum. Note that in each case smaller maturing oocytes are present in addition to a group of larger gametic cells. In mature ovaries, the group of larger gametic cells is comprised of mature oocytes; and in ripening ovaries, the group of larger gametic cells is comprised of ripening oocytes. In ripe ovaries, the larger gametic cells are ripe eggs.
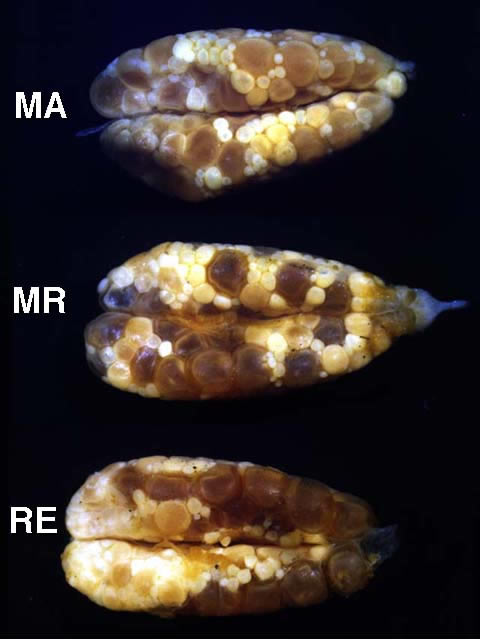
Figure 6
Variation in mature ovaries of Etheostoma caeruleum females.
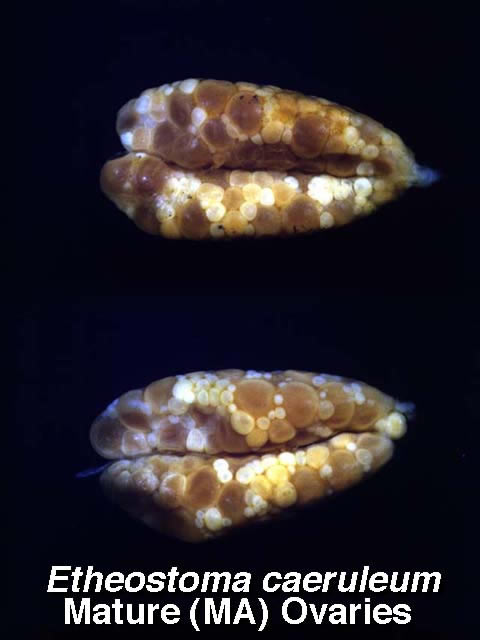
Figure 7
Variation in ripening ovaries of Etheostoma caeruleum females.

Figure 8
Variation in ripe ovaries of Etheostoma caeruleum females.
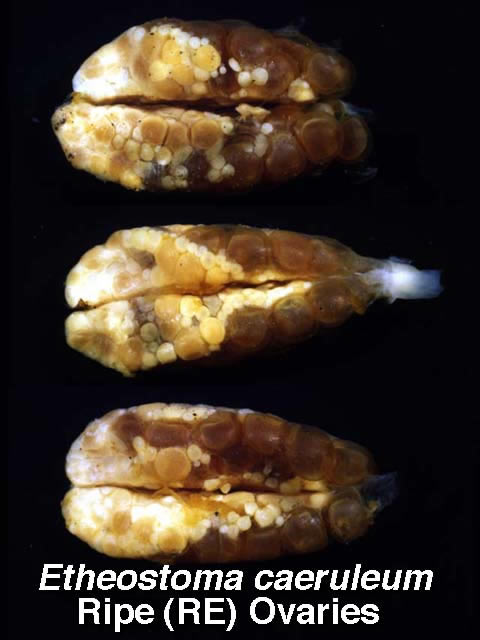
Figure 9
Mature, ripening and ripe ovaries of Etheostoma simoterum females.