Current research projects
Understanding vibrational energy flow in molecules, resolved in time and space, is one of the challenges of physical chemistry. It is important for a wide range of fields, spanning from biochemistry, chemistry, and molecular electronics to novel materials and technology. In chemical reactions, vibrational energy of the reactants is required for reaching the transition state, whereas the removal of the excess energy from the products makes the reaction irreversible. Heat management problems occur in various areas of technology and science. Efficient removal of excess vibrational energy is crucial for the longevity of the elements for molecular electronics. Creating compact systems with controllable energy transport properties, including the cases of high, low, anisotropic, and controlled conductivities, is very desirable.
Earlier, Rubtsov group developed a new approach to 2DIR spectroscopy that uses vibrational energy transport in molecules to enhance the cross-peak amplitudes and therefore increase by several fold the range of distances experimentally accessible by 2DIR for structural measurements. The method is named relaxation-assisted 2DIR (RA 2DIR), see for example Acc. Chem. Res. (2009), http://pubs.acs.org/doi/pdfplus/10.1021/acs.accounts.5b00299 and Proc. Natl. Acad. Sci. U.S.A., (2007) 104, 14209-14214. http://www.pnas.org/content/104/36/14209. We have demonstrated that RA 2DIR can result in ca. 30-fold cross peak amplification and enables assessing distances up to 60Å. The RA 2DIR method is also suitable for assessing energy transport dynamics.
Heat transport in materials often occurs via acoustic phonons, low-frequency delocalized vibrational modes. Their delocalization greatly exceeds interatomic distances, allowing the formation of free propagating wavepackets. High-frequency modes in molecular materials, optical phonons, are typically much more localized than the acoustic modes, which limits their contribution to heat transport and prevents their use for efficient energy transport in such systems. However, energy transport through molecular backbones often occurs using high-frequency vibrations. Such vibrational modes can deliver much larger energy quanta. In addition, we found conditions at which such energy transport can be very fast and efficient, as proceeds in ballistic fashion. One can imagine a pack of energy (a wave packet) propagating via a molecular backbone at constant velocity. Moreover, the transport speed can be different within the same molecular chain depending on the transport initiation mechanism. Figure 1 summarizes results of the energy transport velocities via alkane chains.
Fig. 1. Energy transport speeds in alkane chains with initiation by azido-group excitation (blue and green), and carbonyl mode excitation (red). The chain bands responsible for the transport are indicated with matching color. [Rubtsova et al. Acc. Chem. Res., (2015), 48, 2547-2555; http://pubs.acs.org/doi/pdfplus/10.1021/acs.accounts.5b00299]
Current directions include studies of the transport via alien groups positioned in the middle of a regular chain (Fig. 2). Reformation of a vibrational wavepacket at the alien group and was recently observed (see Fig. 2 caption), which enables perturbing the transport and may permit its control via external stimuli.
Fig. 2. Results of energy transport via alien amide group positioned in the middle of a regular alkane chain. Reformation of a vibrational wavepacket at the amide is observed. [L. Qasim et al. J. Phys. Chem. C, (2019) 123(6) 3381; https://pubs.acs.org/doi/full/10.1021/acs.jpcc.8b11570]
The instrument design was supported by the National Science Foundation under grant number (NSF CHE-1040491). The Louisiana Board of Regents (Grant LEQSF(2011-12)-ENH-TR-29) supported acquisition of the fs Ti:Sapphire laser system used for the instrument.
Principles of the 2DIR method
Two-dimensional infrared (2DIR) spectroscopy emerged about a decade ago as a new tool for measuring three-dimensional structures of molecules in solution and in solid phase. The method measures pair-wise couplings (interaction strengths) of vibrational modes in molecules – the coupling strength reports on the distance between the vibrating groups, thus producing a bit of structural information. The coupling is determined from the strength of the cross-peak in the 2DIR spectrum; by combining many bits of structural data the 3D structure of the molecule can be determined. Many vibrational modes in molecules are localized on particular groups, which make them perfect reporters for the molecular structure. 2DIR spectroscopy has demonstrated its power for measuring structures of numerous compounds including drugs, peptides, transition metal complexes, and recently proteins. Wider implementation of this powerful method is constricted by absence of a commercial instrument capable of accessing the entire 2DIR spectrum. Recent developments of Martin Zanni group at the University of Wisconsin-Madison made a single-color 2DIR spectrometer commercially available. The spectrometer uses a novel pulse shaper and is capable of accessing any peaks in the diagonal region. Because typical mid-IR laser pulses have a width of ca. 200 cm-1 the single-color approach permits measuring structural constraints based only on modes that are close in frequency. The dual-frequency 2DIR instrument built at Tulane University uses two independently tunable mid-IR pulses and has no limitations on the mode frequencies. Moreover, often unwanted diagonal peaks can be strongly reduced or fully eliminated using dual-frequency 2DIR. We have designed and built the first in the world fully automated dual-frequency 2DIR instrument that permits measuring any cross peak within a spectral range from 800 to 4000 cm-1.
Basic characteristics of the instrument
The instrument is designed to measure weak cross peaks and its sensitivity, therefore, is of great importance. We have implemented the most sensitive approach to measure cross peaks: a three-pulse photon-echo method with heterodyned detection. As a result, the instrument has superior sensitivity that reaches 10-3 cm-1 in measured couplings. The high sensitivity is achieved by a combination of spectral interferometry, phase cycling, and closed-loop active phase stabilization accurate to 70 as. The automatic frequency tuning is achieved by implementing beam direction stabilization schemes for each mid-IR beam, providing beam stability better than 50 μrad and a novel scheme for setting the phase-matching geometry for the mid-IR beams at the sample. The instrument is fully computer controlled and, therefore, can be used by non-specialists in ultrafast spectroscopy.
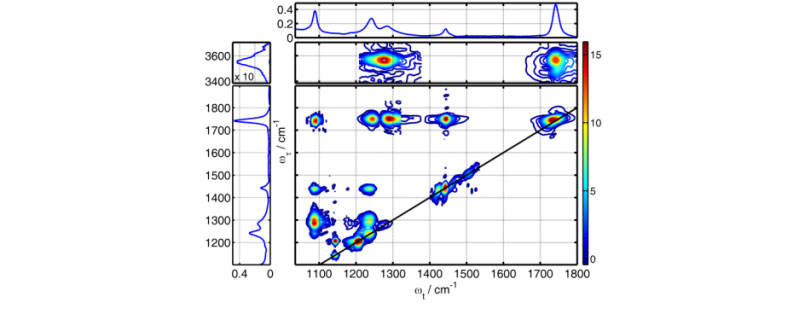
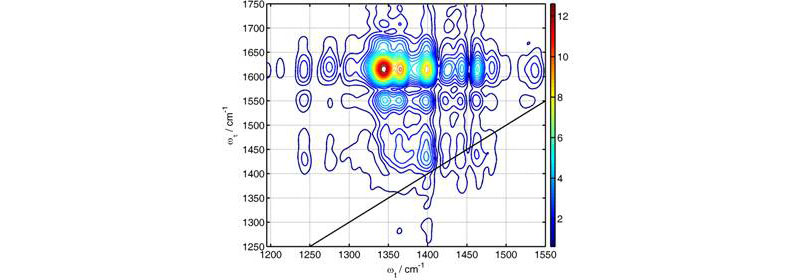
Figure 1 shows an example of 2DIR spectrum measured by the instrument. The acquisition of the entire 2DIR spectrum is performed in a fully automatic regime. The spectrum is composed of several pieces, each covering about 150x250 cm-1 (the spectral range covered by a 64-element array detector depends on the wavelength and on the monochromator grating used). Spectral acquisition of each region took ca. 3-5 min. Transition from one spectral region to another also requires about 3-5 min. This time is needed to change the central frequencies of the two mid-IR beams, stabilize their directions, change the geometry of the beams interacting with the sample, change the oscillation amplitude for the phase-cycling devices, change the monochromator wavelength, change the delay between mid-IR pulses of different frequencies, adjust the intensity of the local oscillator, and recover a π/2 phase shift between two detectors for active phase stabilization (Image 3). Most of the changes are made automatically, but some, such as setting the monochromator wavelength and intensity of the local oscillator, require input of the operator at the computer. The most time consuming step is currently the stabilization of the beam directions, which takes about one and a half minutes for each beam.
Research problems assessable with the instrument
Structural features of small molecules (Maekawa, Sul et al. 2013), including drugs, transition metal complexes (Keating, McClure et al. 2010), etc., structures of peptides (Sengupta, Maekawa et al. 2009; Backus, Bloem et al. 2010) and ligand binding in proteins structures (Fang, Baumann et al. 2008; Bloem, Koziol et al. 2012) of molecules adsorbed on nanoparticles (Donaldson and Hamm 2013) and monolayers (Rosenfeld, Nishida et al. 2013), aggregates in solution, etc. The structural constraints include couplings strengths of vibrational modes, angles between the interacting vibrational transition moments, backbone connectivity patterns (Naraharisetty, Kasyanenko et al. 2008), etc. Dynamics of vibrational energy transport in molecules can be studied (Kurochkin, Naraharisetty et al. 2007; Rubtsov 2013).
As new approaches are being developed, the ability of 2DIR to target new research questions will certainly expand. Research proposals based on unsolved scientific questions are encouraged; such topics can go beyond the already demonstrated applicability of 2DIR spectroscopy.
Access to the instrumentAlthough fully automated, the DF 2DIR instrument is a complex device. Therefore, access to it will be provided via collaborations. Interested parties are encouraged to contact I. Rubtsov to discuss the feasibility of the project and other details. Initially the measurements will be performed by the graduate students of Rubtsov lab. However, direct involvement of collaborators in using the instrument is encouraged. Training will be provided by the members of Rubtsov group.
One of the first research projects started with the instrument is in collaboration with Prof. Jonathan Sessler (UT Austin), who visited the PI's lab in November 2013. The project is devoted to understanding the structures of aggregates of asymmetrically substituted pyrenes in solution, which form very specific and compact packs of molecules in the solid state. Dr. Sessler lab has recently found that such compounds can be used for chemical communication in molecular-scale logical devices.
Disclaimer: "Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation."
(funded by NSF grant CHE-0750415 in 2008-2011)
In 2006, we proposed a new approach to 2DIR spectroscopy that uses vibrational energy transport in molecules to enhance the cross peak amplitudes and therefore increase by several fold the range of distances experimentally accessible by 2DIR for structural measurements. The method is named relaxation-assisted 2DIR (RA 2DIR), see for example Acc. Chem. Res. (2009), 42, 1385 and in “Ultrafast Infrared Vibrational Spectroscopy.” Ed. M. Fayer, Taylor and Francis, 2013, p. 333. We have demonstrated that RA 2DIR can result in 27-fold cross peak amplification, thus allowing assessment of distances up to 60Å.
Because the cross-peak amplification in RA 2DIR depends on the efficiency of the energy transport in molecules, the method also provides the unique opportunity of studying energy transport on a molecular scale. The ability to predict energy transport dynamics in molecules would bring RA 2DIR to the level of an analytical technique.
One of our research directions targets the understanding of energy transport dynamics and its relation to molecular structure. In collaboration with Prof. Alex Burin at Tulane, we are also developing theoretical models to describe such dynamics on a molecular scale (e.g. J. Phys. Chem. A, (2013) 117, 315).
Diffusional vs. Ballistic Energy Transport
There are two general limits for the energy transport: One is described by a diffusion-like heat conduction equation, while another assumes vibrational wavepacket propagation and results in ballistic energy transport. Both regimes can be found for energy transport in macroscopic and mesoscopic samples in the condensed phase; the ballistic transport requires delocalized vibrational states in the sample and can be observed for transport distances less than or comparable to the mean-free-path length of the corresponding phonon. Both acoustic and optical bands can transfer energy ballistically, while the dispersion relations for the acoustic band(s) favor wavepacket propagation with limited broadening.
We have recently discovered a ballistic energy transport in molecules in solution to large distances (e.g. Proc. Natl. Acad. Sci. U.S.A., (2012) 109, 1413 and Chem. Phys. DOI: 10.1016/j.chemphys.2013.01.026). Energy transport along polyethylene glycol (PEG) and perfluoroalkane chains was investigated and found to occur with a constant speed of ca. 500 m/s (5 Å/ps) and the transport has been detected for distances up to ca. 60 Å. The goal of this project is to investigate further the ballistic energy transport in molecules and find possibilities of controlling (directing) the energy flow on a molecular scale. A variety of potential applications can be envisioned, including novel signal transduction schemes with molecular waveguides that use vibrational energy propagating at high speed and opportunities of delivering packets of energy to initiate chemical reactions.
(NSF CHE-1012371)
The project aims at investigating how to modulate (control) electron tunneling pathways using mid-IR radiation and seeking an understanding of inelastic tunneling interactions in molecules. It is highly interdisciplinary project involving laboratories of experimental physical chemistry (us), inorganic/organic chemistry (laboratories of Profs. J. Sessler at UT Austin, R. Schmehl at Tulane, and M. Therien at Duke) and theoretical chemistry (Prof. D. Beratan at Duke).
At the core of quantum mechanics is the notion of representing different possible outcomes by the coherent superposition of probability amplitudes. In chemistry, coherent superpositions underlie chemical bonding, reactivity, and spectroscopy. These fundamental issues are particularly important for electron-transfer reactions. For example, nonadiabatic electron-transfer reactions involve the coherent tunneling of electron amplitude through a chemical “linker”. These tunneling reactions lie at the heart of solar energy conversion, bioenergetics, nanoscale information processing, and catalysis. We have recently discovered that IR excitation may be used to manipulate the propagation of electron amplitude from donor to acceptor species in molecules (J. Am. Chem. Soc. (2009), 131, 18060). The objective of this research program is to explore new experimental and theoretical strategies that will enable the active control of electronic amplitude propagation in molecules through IR radiation.
The experimental approaches used in this study includes a variety of time resolved spectroscopy methods such as three-pulse (UV or VIS) pump – mid-IR pump – (mid-IR or VIS) probe, UV-VIS transient absorption, (UV or VIS) pump – mid-IR probe, dual-frequency 2DIR, and triggered 2DIR spectroscopies.
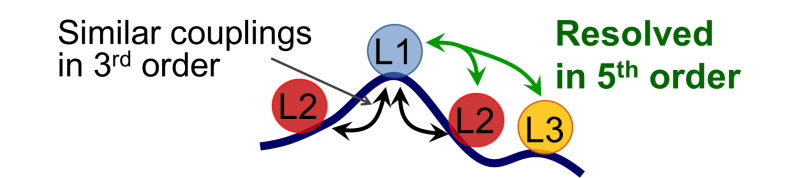
5th order triple-frequency infrared spectroscopy has potential to enhance the spectral resolution and selectivity compared to the linear and 3rd order spectroscopies. The advantages of higher order IR spectroscopy for structural measurements can be illustrated using the analogy with multi-pulse NMR spectroscopy. If one uses a 900 MHz NMR machine instead of a 300 MHz one, the resolution is enhanced by 3 times. When an additional pair of radio-frequency pulses is implemented (going to 5th-order compared to 3rd-order, for example) the spectral resolution is enhanced by at least 100 times. Triple-frequency 5th-order spectra in principle permits enhancing spectral resolution in structural measurements. The goal of this project is to develop triple-frequency infrared spectroscopy and demonstrate its advantages.
Alzheimer's Disease
Soluble amyloid-b peptides (Ab) are found in all biological fluids of the human body as well as in amyloid plaques in brain, which are considered to be neurological indicators of Alzheimer’s Disease (AD). Despite significant progress in understanding the mechanisms associated with the disease on a molecular level, the physiological roles of b-amyloid precursor protein (APP) and especially of Ab are not well understood. Several hypotheses were proposed assigning various regulating functions to Ab, such as involvement in lipid, metal-ion, and cholesterol homeostases. We are using a novel structural technique, two-dimensional infrared (2DIR) spectroscopy, particularly the relaxation-assisted 2DIR method (recently developed in the laboratory) for addressing the questions of the Ab homeostasis.
Conformational Dynamics in Complex Molecular Systems (supported by BoR)
We are interested in following structural changes in molecules (especially proteins and enzymes) induced by a certain event, such as photon absorption, ligand binding, or pH jump. We have recently developed tools which allow us to study slow conformational processes triggered by light with 2DIR spectroscopy. Because we now can change the delay between the UV/Vis femtosecond “dynamics trigger” pulse and 2DIR pulse sequence (mid-IR range) from tens of fs to a millisecond(!), structural dynamics in this time window will be interrogated.
A collaborative project with Prof. Jeff Rack at Ohio University is directed towards understanding details of photoisomerization process in transition-metal complexes.