Automatic Continuous Mixing was developed by us as a useful technique in studying equilibrium and quasi-equilibrium processes. The motivation is to be able to analyze complex, multi-component systems, increase the resolution of experiments, and allow for higher sample throughput.
It basically includes a programmable mixing pump, operating in step gradient mode or continuous gradient mode, connected to a flexible detector train, with a multi-angle light scattering detector, viscometer, RI and UV detectors.
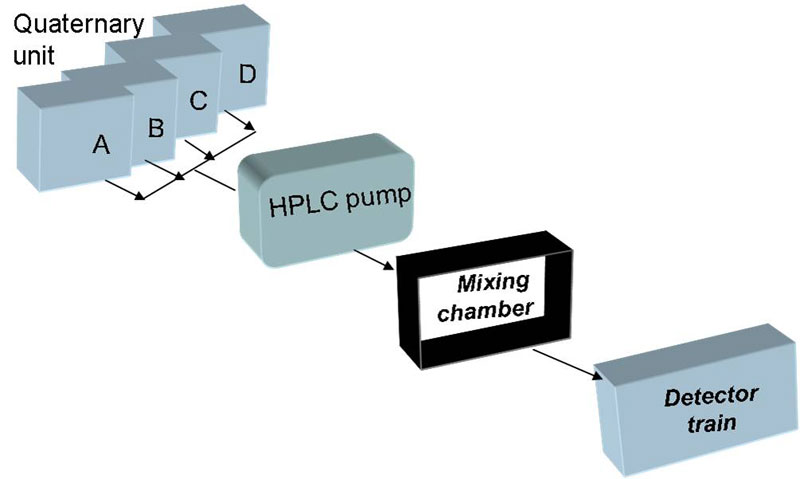
ACM Set-up
Many types of characterizations are possible:
- two-component polymer solutions; Mw, A2 , A3 , [h]w are simultaneously obtained by automatic dilution of a polymer solution into the solvent . Compared to the traditional Zimm plot method, ACM has more accuracy (provides continuum of points), reduces sample preparation time, offers complete information on the polymer sample analyzed.
- polyelectrolyte effects;
- monitoring drug release from nanohydrogel core-shell structures
- association of charged micelles with neutral polymers
- ACM complimentary to ACOMP
- polymer - polymer interactions
- polymer - surfactant interactions;
- surfactants-aromatics;
Typical Results
Polyelectrolyte Effects
The increase in scattering as electrostatic shielding leads to the decrease in A2 and A3, and the contraction of the polyelectrolyte coil, leading to decreased viscosity (G. A. Sorci; W. F. Reed, Macromolecules 2004, 37(2), 554-565).
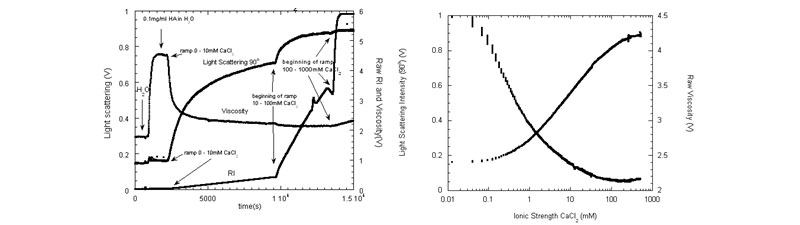
Left: Raw LS at 90°, viscometer, and RI data for a ramp of constant hyaluronic acid concentration from pure water up to 1 M CaCl2.
Right: Raw LS 90° and viscosity vs. [CaCl2].
Association of charged micelles with neutral polymers
Due to the association with SDS micelles, the polymer becomes charged, a 'pseudopolyelectrolyte' (G. A. Sorci; W. F. Reed, Langmuir 2002, 18(2), 353-364).
![[SDS] ramp into PVP salt free solution](/sites/default/files/ACM_pvp_sds.jpg)
[SDS] ramp into PVP salt free solution (c= 0.002 g/cm3 , Mw =2x106 g/Mole)
ACM as a complementary technique for ACOMP
The bimodality in the copolyelectrolyte/neutral polymer blend (4-vinylbenzenesulfonic acid sodium salt/acrylamide) affects the interparicle interactions, seen in the A2 and A3 behavior (A. M. Alb; A. Paril; H. Catalgil-Giz; A. Giz; W. F. Reed, J. Phys. Chem. B 2007, 111, 8560-8566).
![Kcp/I(q=0) vs. cp for a copolyelectrolyte/neutral polymer blend (VB/Aam) produced at 0.1M NaCl, at [NaCl] =0.01M. The inset shows A2 and A3 vs. ionic strength.](/sites/default/files/ACM_vbam_kci.jpg)
Kcp/I(q=0) vs. cp for a copolyelectrolyte/neutral polymer blend (VB/Aam) produced at 0.1M NaCl, at [NaCl] =0.01M. The inset shows A2 and A3 vs. ionic strength.
ACM for interaction of aromatics and surfactants
The striking effect that the aromatic has on intermicellar interactions (left); The abrupt change in A2 as the non-ionic surfactant fills with aromatic molecules and its volume becomes saturated with them (right).
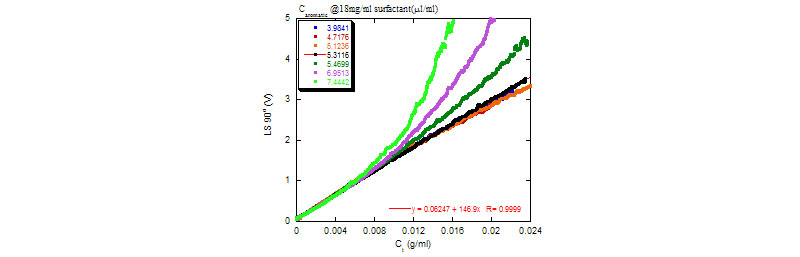
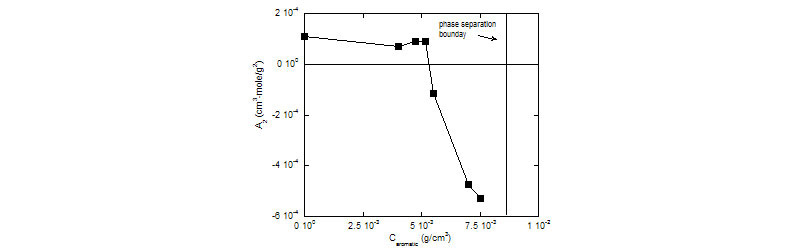
Raw light scattering voltages vs. total (aromatic+surfactant) concentration. A2 for (aromatic+surfactant) solutions vs. Caromatic.
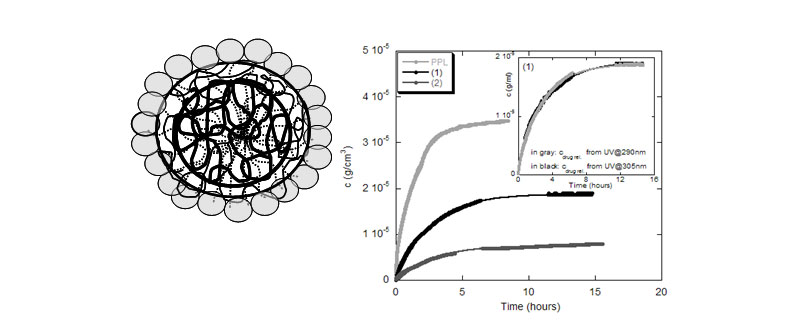
Monitoring drug release from nanohydrogel core-shell structures
The feasibility of poly(acrylonitrile-co-Nisopropylacrylamide), p(AN-c-NIPAM) core-shell hydrogel nanoparticles as drug carrier was investigated. The release of propanolol, PPL from core-shell p(AN-c-NIPAM) (1) and amidoximated p(AN-c NIPAM) (2) was continuously monitored by UV detection with ACM. The hydrophilicity/ hydrophobicity of the composite material can be adjusted by the extent of amidoximation and allows a degree of control over the loading levels of appropriate drug components (N. Sahiner; A. M. Alb; R. Graves; T. Mandal; G. L. McPherson; W. F. Reed; V. T. John, Polymer 2007, 48, 704-711).
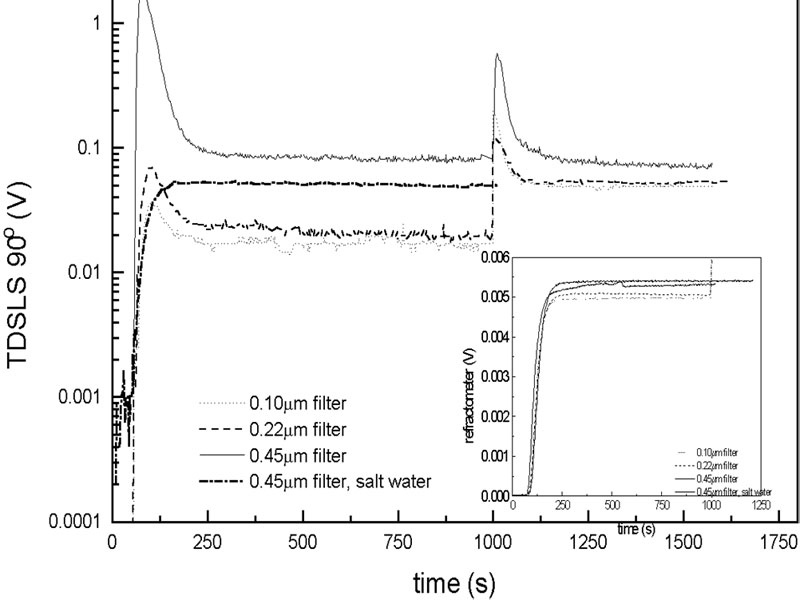
Dissolution of a polyelectrolyte
- Small population of aggregates present in dry powder
- Aggregates dissolve in time
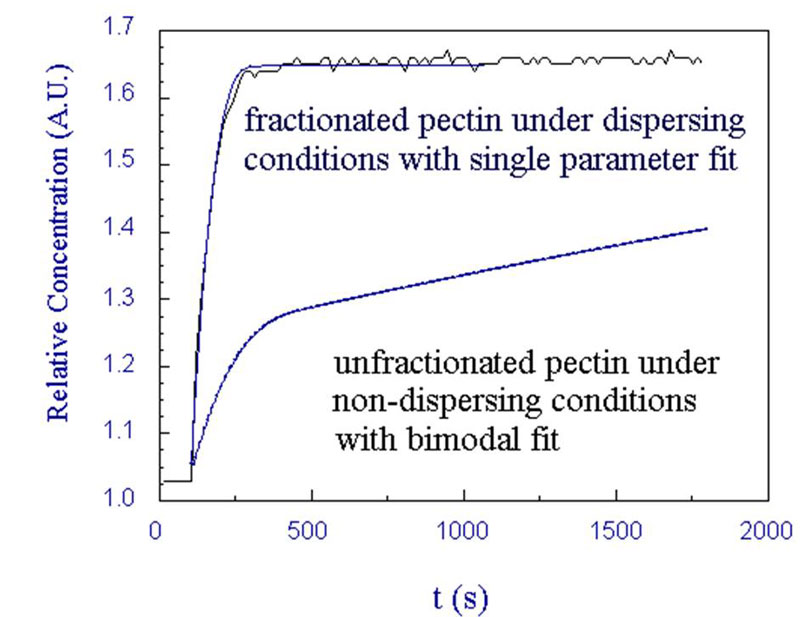
Dissolution of dry polysaccharides
- Origin of poor dissolution due to formation of aggregates
ACM Publications appeared or in press
1. E. Bayly, J. L. Brousseau and W. F. Reed, "Continuous Monitoring of the Effect of Changing Solvent Conditions on Polyelectrolyte Conformations and Interactions", Int. J. of Pol. Charact. and Analysis 2002, 7, 1-19.
2. G. A. Sorci; W. F. Reed, "Electrostatic and Association Phenomena in Aggregates of Polymers and Micelles", Langmuir 2002, 18(2), 353-364.
3. G. A. Sorci; W. F. Reed, "Electrostatically enhanced second and third virial coefficients, viscosity and interparticle correlations for linear polyelectrolytes", Macromolecules 2002, 35(13), 5218-5227.
4. W. F. Reed, "Automatic, Continuous Mixing Techniques for Online Monitoring of Polymer Reactions and for the Determination of Equilibrium Properties", ch. 20, pp. 589-622, Handbook of Size Exclusion Chromatography and Related Techniques", 2 nd Ed., Chi-san Wu, Ed., Marcel Dekker, 2003.
5. G. A. Sorci; W. F. Reed, "Effect of ion type and valence on polyelectrolyte conformations and interactions", Macromolecules 2004, 37, 554-565.
6. N. Sahiner, A. M. Alb, R. Graves, T. Mandal, G. L. McPherson, W. F. Reed, V. T. John, "Core-shell nanohydrogels structures as tunable delivery system", Polymer 2007, 48, 704-711.
