1. Surfactant-free emulsion polymerization
2. Emulsion polymerization with SDS
An original idea was put in practice: use ACOMP to simultaneously measure properties of the dispersed components (emulsions) and their contents (polymer and monomer). dilute one withdrawn reactor stream with aqueous solution to preserve the dispersed (emulsion) phase and measure its characteristics online and the other withdrawn stream with THF to solubilize polymer and monomer.
Challenge – the choice of the most suitable model for the reaction mechanism.
Several approaches are reported in monitoring emulsion polymerization reactions: Raman spectroscopy, NIR spectroscopy, conductivity, calorimetry, densitometry
Drawbacks: calibration models are required; errors in determining measured parameters; difficult to be quantified if more monomers are present; probe contamination
ACOMP contributes to a significant progress in unifying polymer and colloid worlds: Simultaneous monitoring of both polymer and particle characteristics in emulsion polymerization (A. M. Alb and W. F. Reed, Macromolecules 2008)
An original idea was put in practice: use ACOMP to simultaneously measure properties of the dispersed components (emulsions) and their contents (polymer and monomer). dilute one withdrawn reactor stream with aqueous solution to preserve the dispersed (emulsion) phase and measure its characteristics online and the other withdrawn stream with THF to solubilize polymer and monomer.
Raw data and analysis for free radical polymerization of Methyl methacrylate in emulsion at 70C.
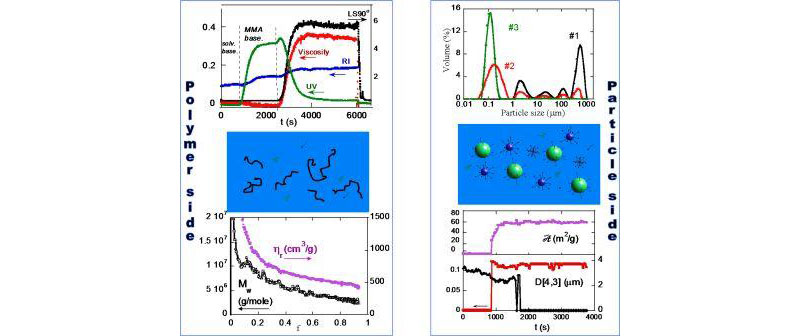
Detectors on the ‘polymer side' included the MALS, viscometer, full spectrum UV/Vis, and RI detectors, as before. The ‘particle side' included a Malvern Mastersizer2000 detector, employing Mie scattering analysis from multi-angle scattering data. On the left side of figure, polymer Mw and hr are determined as functions of conversion, and on the right side, particle size distribution and specific surface area from the Mie scattering analysis are shown.
A number of features are captured by this process:
- multi-phase conversion,
- multimodal mass production,
- the correlation between monomer droplet disappearance and monomer conversion,
- the reaction time and large polymer masses produced.
RESULTS
Surfactant-free emulsion polymerization - from dilute regime to high solid contents (14%): Soap-free emulsion polymerization of butyl acrylate (BA)
a) Polymer side:
![Raw viscosity, light scattering (90) and UV (215nm) data. [BA]=1.11M; [I]=5.586mM](/sites/default/files/em-raw-data_a0711.jpg)
Top: Raw viscosity, light scattering (90) and UV (215nm) data. [BA]=1.11M; [I]=5.586mM.
Bottom: The evolution of Mw with conversion. In the inset to figure, radius of gyration and monomer conversion, f are shown vs. time
b) Particle side:
Large monomer-containing droplets (microns) gradually cede their contents to a growing population of polymer- containing micelles of much smaller diameter (fractions of a micron
![The evolution of the specific surface area A (upper) for two BA polymerization reactions. The volume weighted mean diameter, D[4,3] for two modes in the particle size distribution (lower) for the 14% BA.](/sites/default/files/particle-size_comp.jpg)
![Cryo-TEM image for BA surfactant–free polymerization reaction ([BA]=0.268M)](/sites/default/files/cryo-pba.jpg)
Top: The evolution of the specific surface area A (upper) for two BA polymerization reactions. The volume weighted mean diameter, D[4,3] for two modes in the particle size distribution (lower) for the 14% BA.
Bottom: Cryo-TEM image for BA surfactant–free polymerization reaction ([BA]=0.268M)
Emulsion polymerization with surfactant added (SDS) - from dilute regime to high solid contents (35%): Soap-free emulsion polymerization of methy methacrylate (MMA) - polymer side
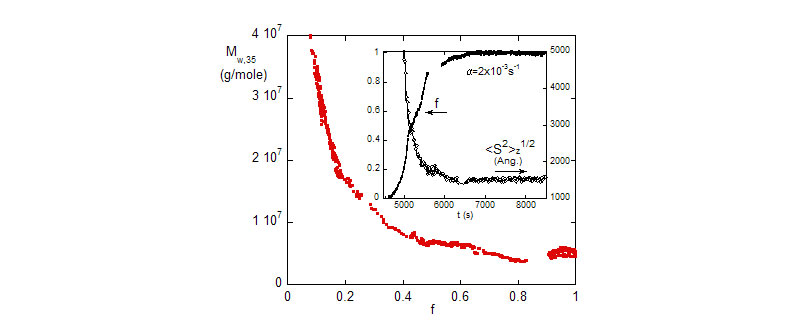
Kinetic and molar mass analysis show that free radical polymerization of MMA and BA in emulsion approximately resemble free radical polymerization in homogeneous phase, with some notable differences in details.
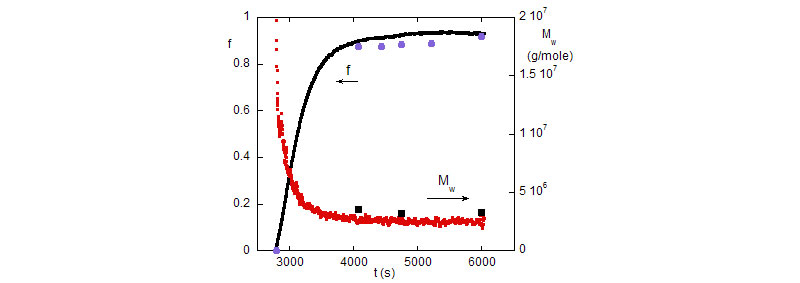
Top: The evolution of the fractional monomer conversion f, and the polymer mass, Mw during the polymerization reaction (0.45M). Discrete points are SEC results from manually withdrawn reaction aliquots.
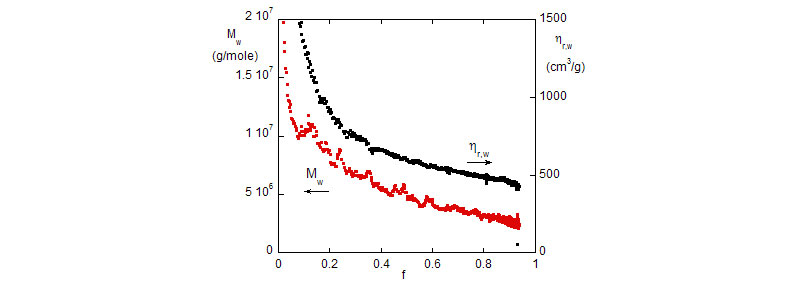
Bottom: Weight average reduced viscosity, hr,w and Mw vs. monomer conversion.
Emulsion polymerization with surfactant added (SDS) - chain transfer effect: Emulsion polymerization of MMA and BA, respectively (~15%)
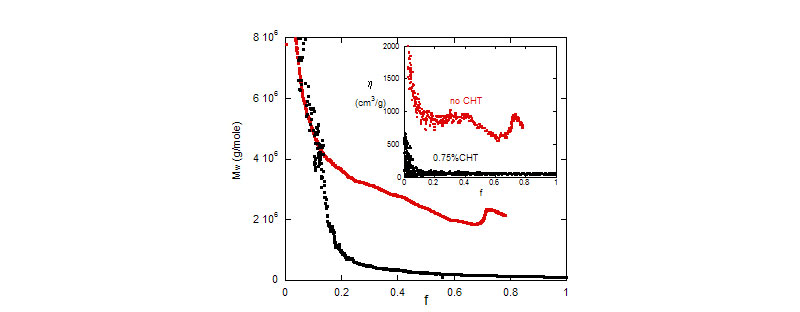
Top: MMA polymerization reactions - Mw vs. conversion for reactions with and without chain transfer agent. In the inset , reduced viscosity hr,w as functions of conversion for the same reactions.
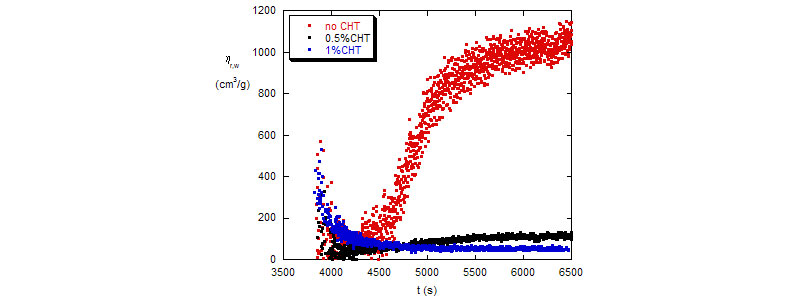
Bottom: BA polymerization reactions - hr,w as functions of time for reactions with different amount of transfer agent.
Emulsion Copolymerization - computing concentration of each comonomer during the reaction allows composition drift and distribution to be determined
INVERSE EMULSION COPOLYMERIZATION
- First heterogeneous polymerization reaction monitored by ACOMP (A. M. Alb, R. Farinato, J. Calbick, W. F. Reed, Langmuir 2006, 22, 831-840)
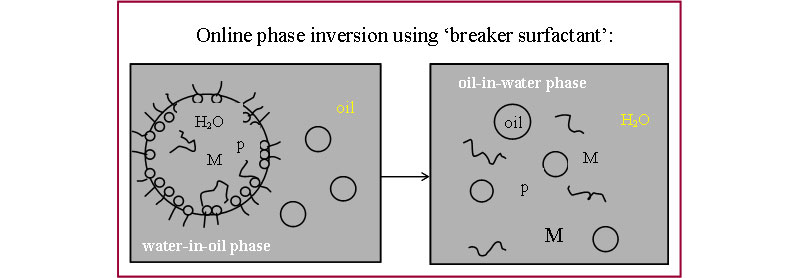
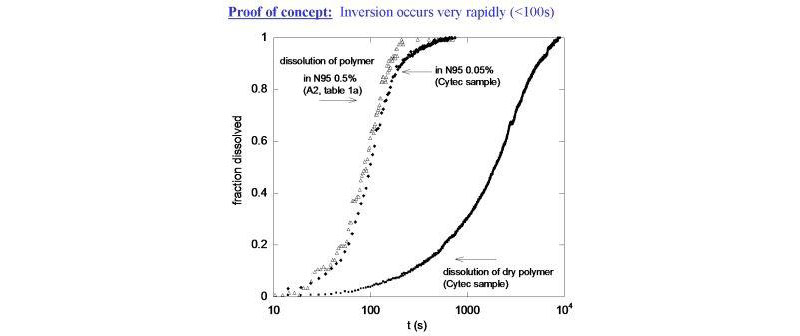
To make absolute measurements on a complex system containing oil, water, monomer, polymer, initiator, and surfactant,special means of online sample “conditioning” and data interpretation needed to be found. Stated simply, the monomeric and polymeric contents of the inverse emulsion/latex must be “spilled” out from the discrete phase droplet in a period of seconds, and the resulting detectors signals must be interpreted to allow differentiating among the complex mixture of components.
This work is meant to:
- establish means of continuously inverting and conditioning the reactor liquid, determining the type of behavior that occurs in the multicomponent system upon conditioning, and how the relevant components can be tracked with the detector train;
- make initial ACOMP measurements with the goal of obtaining preliminary kinetic data.
- Strategy: use of HLB (high lypophilic balance) surfactant (or 'breaker surfactant') to invert the water-in-oil inverse emulsion droplets used in polymerization to an oil-in water system.