Goals
- simultaneous, model-independent determination of the average composition;
- simultaneous, model-independent mass distribution without requiring measurements in different solvents;
- model-dependent instantaneous and cumulative composition and mass distribution;
- monomer reactivity ratios;
- model-dependent sequence length distribution, using the Mayo-Lewis theory
General features
Composition and sequence length distribution are important in determining the 'blockiness' of the copolymer, microscopic morphology, and macroscopic properties, including solubility, blending and other thermodynamic behavior. Understanding the copolymerization propagation kinetics facilitates the modeling and hence the control of the composition and sequence distribution. Varying the monomer feed fractions allows a specific copolymer composition to be targeted and gives predictions for the sequence length distribution. Therefore, access to a large variety of copolymer structures is provided.
1. Terminal model - assumes that the chemical reactivity of a propagating chain depends only on the monomer unit at the growing end. The rates of disappearance of the monomers are given by:
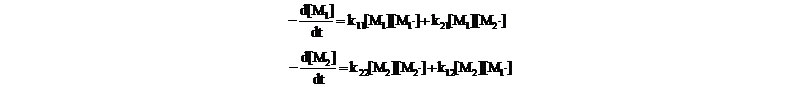
- Under QSSA (d[M1]/dt~0, d[M2]/dt~0), an expression for copolymer equation was obtained:
![]() , r1=k11/k12, r2=k22/k21
, r1=k11/k12, r2=k22/k21
- If r1 >1, M1 preferentially adds M1 units.
- If r1 <1, M1 preferentially adds the other monomer (M2) units.
- If both r1 and r2 >1 a tendency to form a block copolymer is observed.
- If r =0 alternating copolymers are obtained.
A copolymerization is termed ideal when r1*r2 =1 (both of the propagating species show the same preference for adding one or the other of the two monomers. For r1= r2=1 the two monomers show equal reactivities toward both propagating species and the copolymer composition is the same as the comonomer feed, the monomers being randomly incorporated in the copolymer chain.
The instantaneous mole fraction of [M1] incorporated, F1 is given by
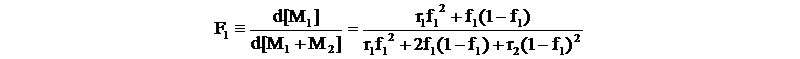
f1=[M1]/([M1]+[M2]), f2=[M2]/([M1]+[M2]
2. Penultimate model - assumes that the rate of propagation is affected by both the terminal and penultimate units of a polymer radical; eight propagation equations are involved,with the four reactivity ratios:
r1=k111/k112, r1'=k211/k212, r2=k222/k221, r2'=k122/k121
Each monomer is characterized by two reactivity ratios: r represents the propagating species in which the penultimate and terminal units are the same and r' represents the propagating species in which the penultimate and terminal units differ.
The copolymer composition becomes:
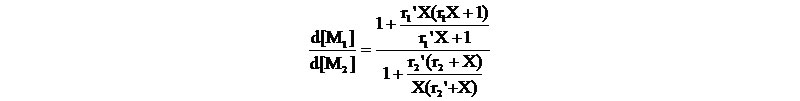
, X =[M1]/[M2]
Typical Results
Continuum set of data points from various detectors allow accurate information to be obtained.
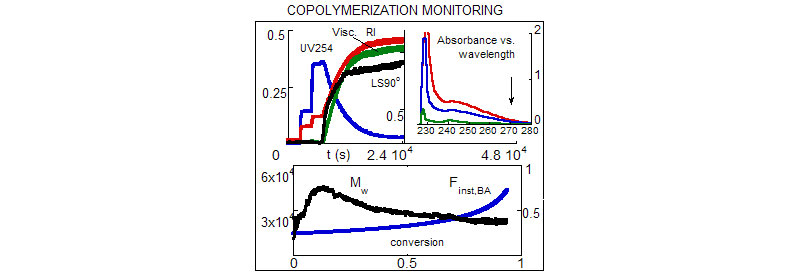
Differences in differential refractive index and ultraviolet absorption of comonomers in their monomeric and polymeric forms, were exploited to obtain the monomer and polymer composition as a function of time and, simultaneously, total monomer conversion.
Use of simultaneous multi-angle light-scattering data, together with the detailed composition data allowed the evolution of Mw to be followed, in model independent fashion, avoiding the use of traditional methods of making scattering measurements in three different solvents.
Valuable information on the copolymer intrinsic viscosity and a cross-check on the masses derived from light-scattering behavior were obtained.
Reactivity ratios were computed using two distinct methods; initial kinetics, and a parameter space search.Instantaneous and cumulative sequence length distributions and averages were determined based on the Mayo-Lewis model.
- A recent advance in ACOMP-Discrimination of Comonomers of similar spectral characteristics
- ACOMP allows new monomers to be characterized during copolymerization
- Other copolymerization system studied
1. A recent advance in ACOMP - Discrimination of comonomers of similar spectral characteristics: Butyl acrylate (BA)/Methyl Methacrylate (MMA) copolymerization
Full spectrum UV (photodiode array) is used with error minimization between experimental and calculated spectra; no modeling, no empirical correlations, just linear superposition of spectra
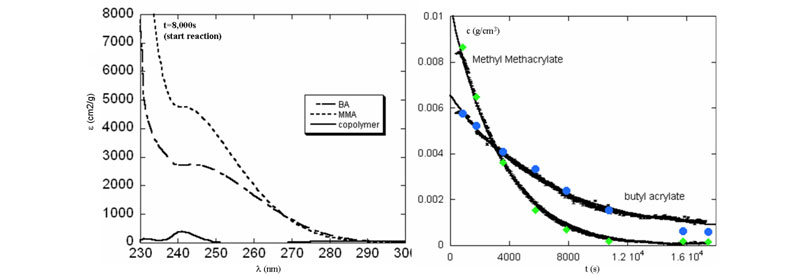
Left: Average extinction coefficient basis spectra for MMA, BA and the copolymer.
Right: Computed comonomer concentration in detector train (in black). GPC results from samples taken during reaction are shown as well (blue and green).
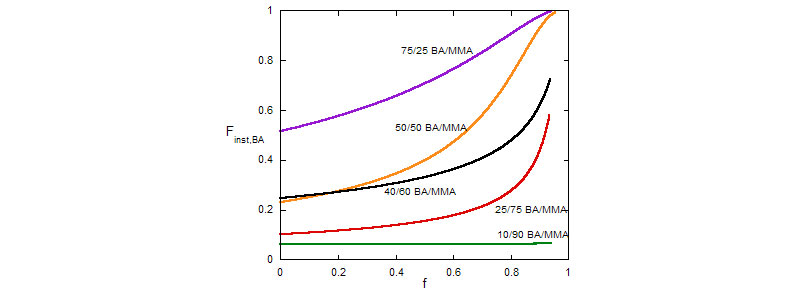
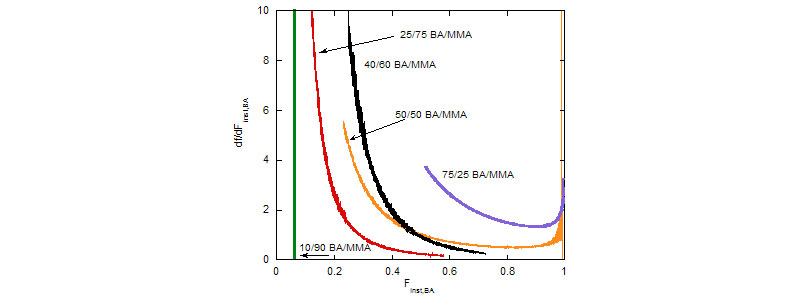
Composition drift (Top) and average composition distribution (Bottom) during MMA/BA free radical copolymerization reactions
(A. M. Alb, P. Enohnyaket, M. F. Drenski, A. Head, A. W. Reed, W. F. Reed, Macromolecules 2006, 39, 5705-5713)
2. ACOMP allows new monomers to be characterized during copolymerization: N-methacryloxysuccinimide (MASI) with methyl methacrylate (MMA) and butyl acrylate (BA)
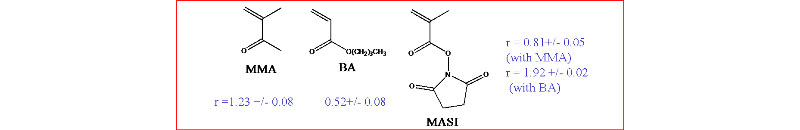
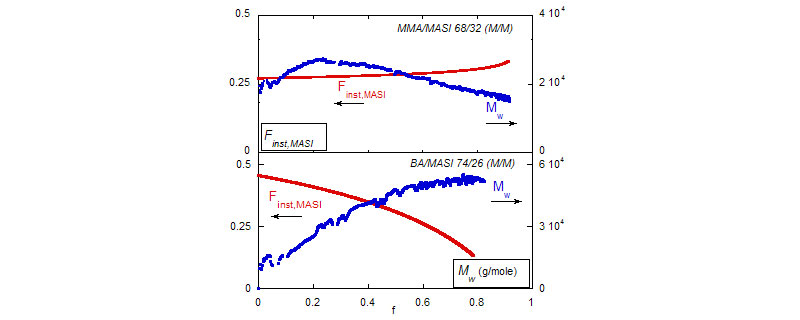
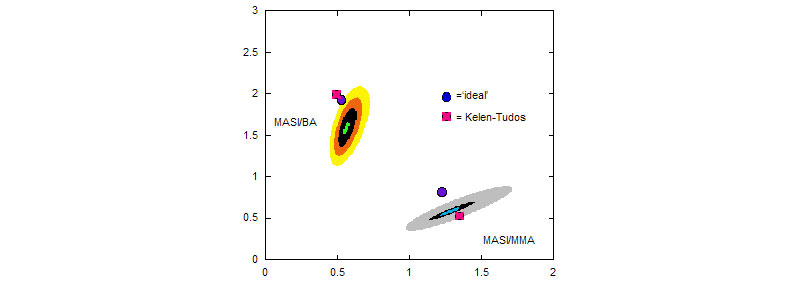
Top: ACOMP allows to observe opposite trends in the evolution of the copolymer mass, Mw and of the composition drift, Finst, MASI as MASI copolymerize with MMA and with BA, respectively.
Bottom: comonomer reactivity ratios given by ACOMP
(A. M. Alb, P. Enohnyaket, M. F. Drenski, R. Shunmugam, G. N. Tew, W. F. Reed, Macromolecules 2006, 39, 8283-8292)
3. Other copolymerization system studied: styrene (Sty)/methyl methacrylate (MMA) copolymerization reaction
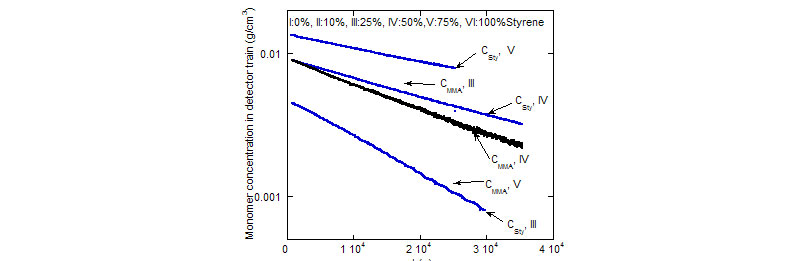
Top: Based on raw data furnished by ACOMP (RI and UV), comonomer concentrations for several experiments with different initial composition (semi-log plot)are determined.
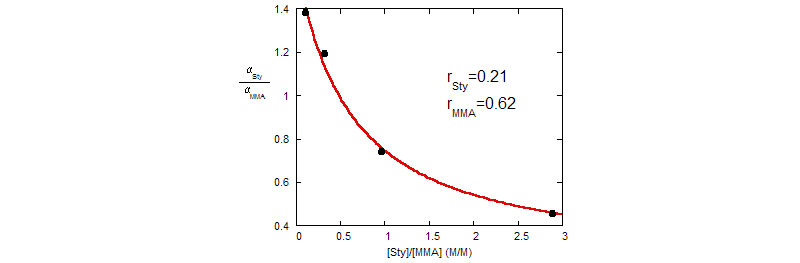
Bottom: Determination of the comonomer reactivity ratios rSty and rMMA from styrene and MMA rate constants
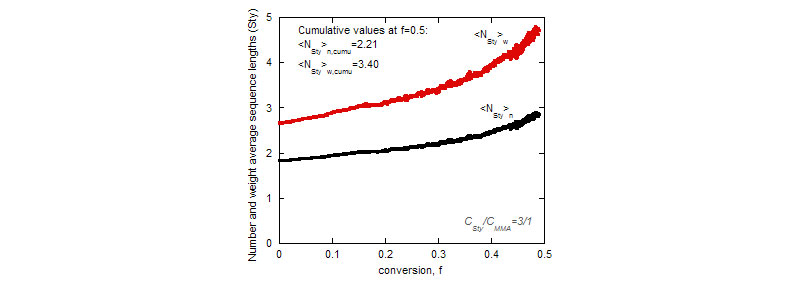
Top: Instantaneous number and weight-average sequence lengths for styrene as functions of conversion.
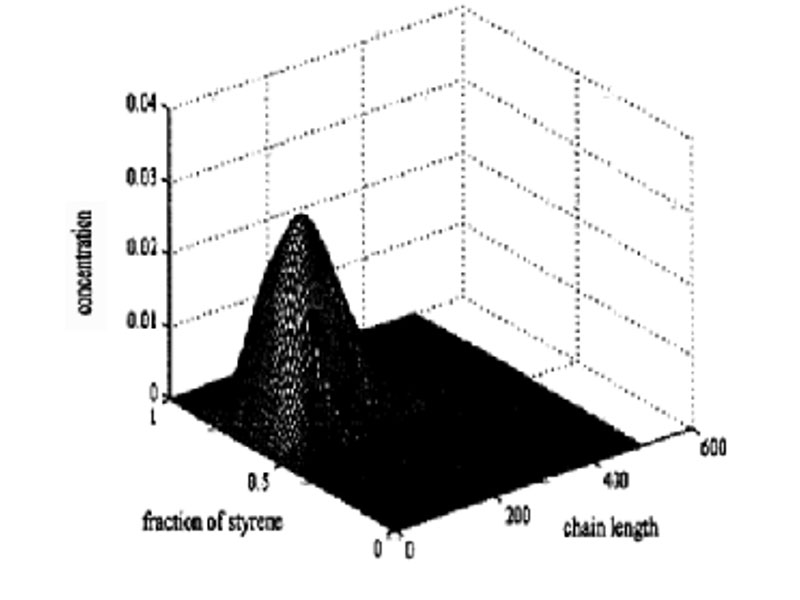
Bottom: 3-D bivariate distribution for experiment V (sty/MMA=3/1), using the Stockmayer bivariate distribution.
(H. Catalgil-Giz, A. Giz, A. M. Alb, A. Oncu1 Koc,W. F. Reed, Macromolecules 2002, 35, 6557-6571)
