ACOMP is used to monitor the synthesis of copolymeric polyelectrolytes: Unified understanding of copolyelectrolyte properties via monitoring of their synthesis, and subsequent endproduct characterization
1. Acrylamide (Aam)/ Vinyl benzene sulfonic acid sodium salt (VB) copolymerization
Large composition drift leads to bimodality - a first step in producing polymer blends: copolyelectrolyte and polyacrylamide.
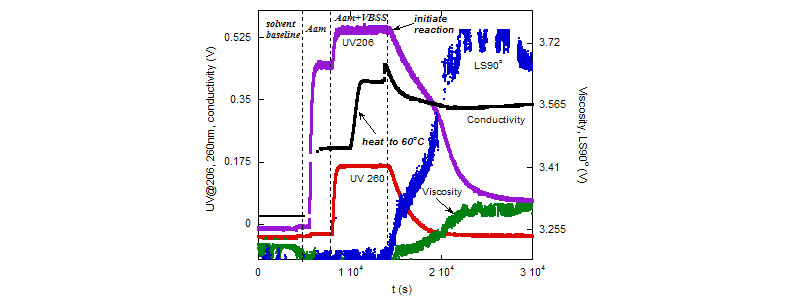
Top: Raw ACOMP data for 10%/90% (g/g) VB/Aam copolymerization in 0.2mM NaCl.
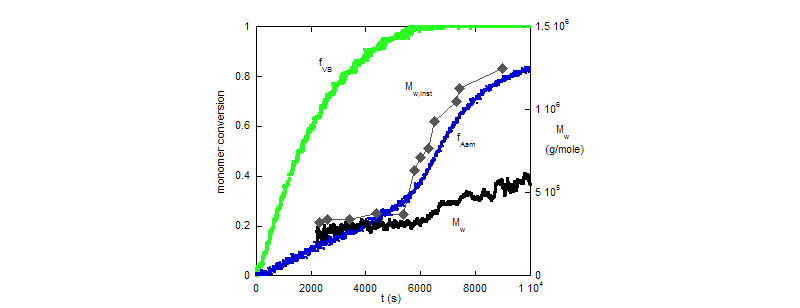
Bottom: Comonomer conversions fVB, fAam, and cumulative and instantaneous weight average mass, Mw, and Mw, inst, respectively.
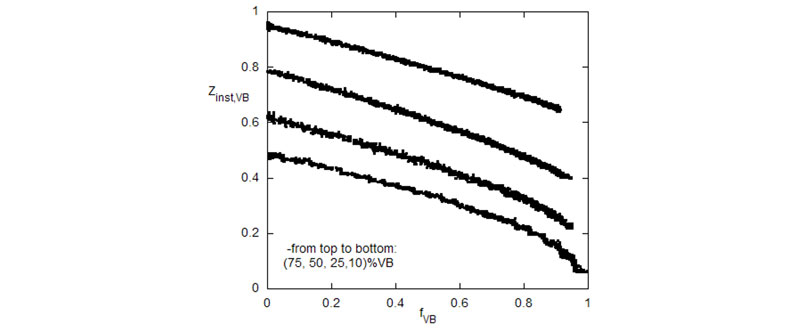
Top: Composition drift of VB vs. fVB for several different starting ratios of VB/Aam.
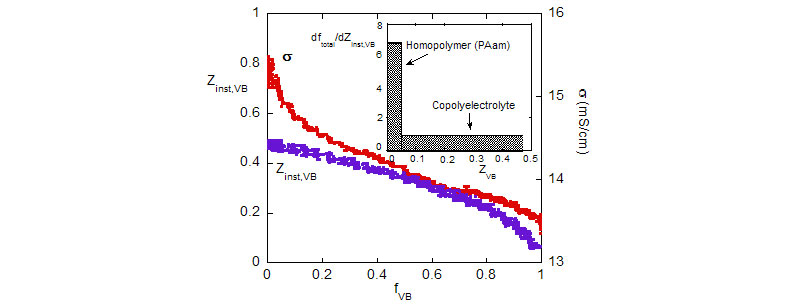
Bottom: Composition drift during polymerization, Zinst,VB vs. fVB, and evolution of conductivity s vs fVB. Inset shows average composition distribution.
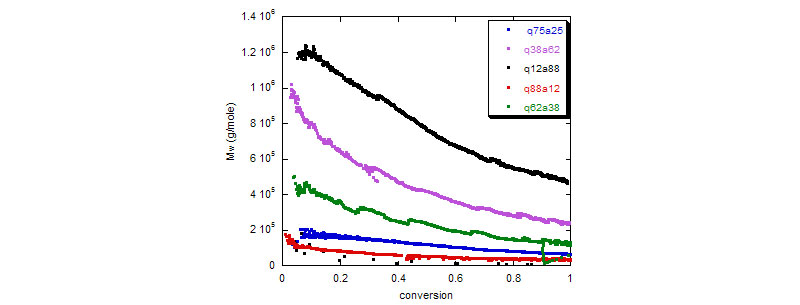
2. Acrylamide (Aam)/[ 2-(acryloyloxy)ethyl ]-trimethylammonium chloride (Q9) copolymerization
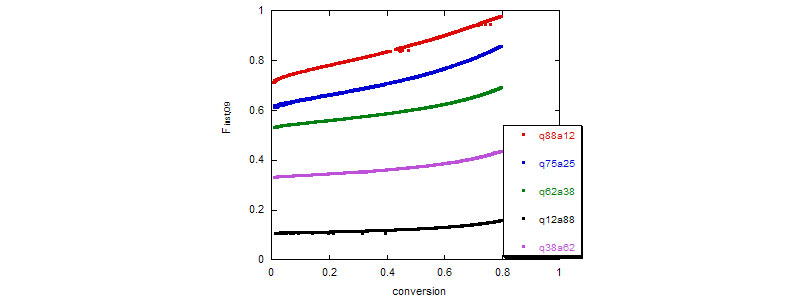
Top: Total fractional composition drift and final Mw vs. Q9 molar %.
Bottom: Mw for several Q9/Aam copolymerization reactions
